Polystyrene is a type of plastic commonly found in plastic cups, containers, and toys. While polystyrene is considered safe under normal conditions and has many applications, including food packaging, disposable dining ware, and construction materials, concerns about its safety have grown over time.
As plastic pollution gains prominence, increasing worries about polystyrene’s safety are emerging, particularly in hot, acidic, or alkaline conditions where polystyrene may leach substances that can be harmful to health and the environment.
Therefore, it is crucial to understand polystyrene’s functions, including its manufacturing processes, environmental impact, and potential health risks. This document aims to summarize these aspects and offer suggestions for addressing these ongoing challenges.
What Is Polystyrene?
Polystyrene (PS) refers to polymers of styrene, with a chemical formula of (C8H8)n. The polystyrene molecular structure is invariant and consists of a patterned carbon chain and methylphenol aromatic rings which provide it clarity and rigidity at room temperature.
Its glass transition temperature is approximately 80-82 ℃, meaning it typically develops cracks at high temperatures. It is often used in disposable containers, which require only rigid surfaces, and foam food boxes.
Polystyrene is extremely common, used in packaging, containers, insulation, disposable tableware, household items, and children’s toys. Because of its versatile functionality, polystyrene is produced in a variety of ways depending on its intended use:
General Purpose Polystyrene (GPPS) has transparent rigidity and can be found in things such as CD case, lab ware, and take-out containers.
High Impact Polystyrene (HIPS) varies by rubber being mixed into polystyrene for greater impact strength and toughness. Typically made for product housings and children’s toys.
Expanded Polystyrene (EPS) is made through a foaming process, producing a low density and excellent cushioning plastic material. The creating foaming process, stiffens the material yet allows for excellent compression, to provide sturdy packaging materials and building insulation.
These properties and strength have made polystyrene important material in many various settings.

Polystyrene: Properties
| Property Category | Description |
| Density | Approximately 1.04 – 1.06 g/cm³ |
| Melting Point | Approximately 240°C |
| Glass Transition Temperature | Approximately 80°C – 100°C |
| Tensile Strength | 30 – 60 MPa |
| Flexural Strength | 50 – 100 MPa |
| Impact Strength | 2 – 5 kJ/m² (non-impact-resistant type) |
| Elongation at Break | 1.5% – 3% |
| Hardness | 70 – 80 (Shore D hardness) |
| Light Transmittance | Approximately 88% |
| Water Absorption | < 0.1% (within 24 hours) |
| Dielectric Constant | Approximately 2.4 – 2.7 (at 1 MHz) |
| Volume Resistivity | >10^16 Ω·cm |
| Thermal Conductivity | Approximately 0.033 W/m·K |
Manufacture And Composition Of Polystyrene
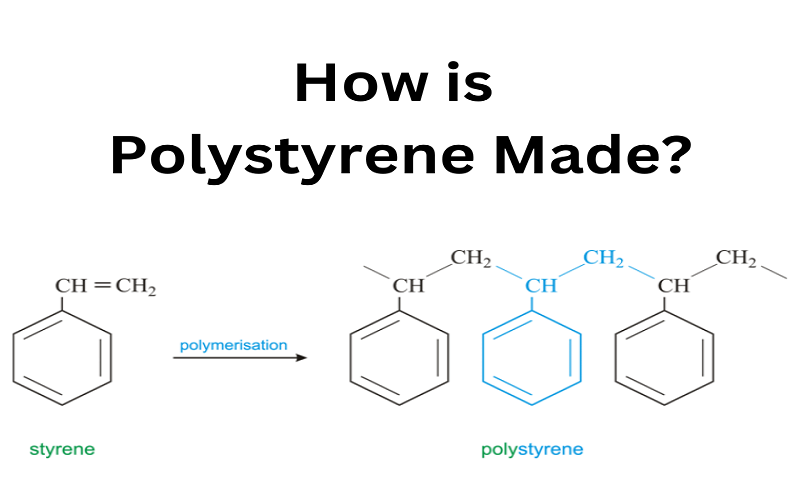
Polystyrene is a synthetic resin or plastic created through the polymerization of styrene monomers. It is a long-chain hydrocarbon polymer, with each repeating unit typically comprised of a polymerized benzene ring.
Polystyrene is clear, rigid, and easy to mold, owing to its molecular structure. Most polystyrene is produced via free radical polymerization reactions involving styrene, initiators (e.g., a peroxide), and heat.
There are several chemicals that are involved in polystyrene production that may affect its properties. Styrene is used as a monomer, while the type and amount of initiator can affect molecular weight, and thus the strength and processing of the produced polystyrene.
In addition, there can be unreacted styrene monomers in the polystyrene product, and these can be hazardous to health and the environment under certain conditions. It is very important to control these factors.
Environmental Impacts Of Polystyrene Plastic
Polystyrene is a plastic that presents serious challenges in terms of degradation due to its relatively high molecular weight and stable molecular structure. Discarded polystyrene products do not biodegrade rapidly in the natural environment, but rather slowly break down into smaller pieces known as microplastics, which can then be ingested by animals and marine life and enter the food chain, introducing risks to ecosystems and potential health hazards for humans.
Furthermore, when polystyrene breaks down in the environment, it may release hazardous chemicals including styrene. Styrene is a toxic substance, and chronic exposure may adversely affect ecosystems and human health.
Polystyrene also has a very low recycling rate, leading to many discarded products ultimately being sent to a waste to energy of incineration. Incineration emits harmful gases including benzene, and may also contribute emissions of other volatile organic compounds, which may contribute to potentially exacerbate air pollution.
In conclusion, polystyrene has large negative environmental implications associated with multi-faceted safety hazards and ecological threats.
The inherent challenges in degrading polystyrene and then the release of dump of hazardous chemicals and substances greatly compounds the environmental challenge posed by polystyrene products.

Is Polystyrene Harmful To Human Health?
Because individuals are paying increased attention to their health and becoming more cognizant of any adverse health effects in association with plastic products, a common question is: “What are the health effects of polystyrene?”
Polystyrene is a cheap and durable plastic that is commonly used as utensils in foodservice operations, takeout containers and hot drink cups.When polystyrene is heated to high temperatures or used with acidic foods, some chemicals may be transferred from the container to the food we are consuming.
Specifically, when heated in the microwave, styrene monomers are more likely to leach from the polystyrene. Styrene is the chemical used to create polystyrene and long-term exposure to it may increase certain cancers, particularly in the lungs and blood. In addition, styrene may adversely affect the nervous system leading to headaches, fatigue, and memory loss.
Aside from styrene, some additives within the polystyrene, including plasticizers and flame retarded, are chemicals that can interfere with hormones in our body and could impact development, reproduction, and the immune system, and increase the likelihood of cancer.
Overall, even though polystyrene is used widely in our daily life, we need to be aware of the potential adverse health effects it may introduce, and especially at elevated heating levels or when reused. It is advised to limit the use of polystyrene food or drink containers when heated,or choose a safer alternative container.

Safer Alternative To Polystyrene
Research demonstrates that polystyrene is generally harmless under ordinary conditions. However, when subjected to heat or in contact with acidic or oily foods, it is capable of releasing some styrene monomers and other chemicals.
Long-term ingestion of these materials might carry risks to health risks such as an increased risk of cancer, potential adverse effects to the nervous system, and endocrine system disruption.
Although styrene is usually relatively low to the polystyrene, many people are concerned about health effects, prompting them to seek alternatives. Here are a few of the materials that may be considered as safer alternatives:
Polylactic Acid (PLA)
PLA is a bioplastic, derived from plant resources, which could be corn starch or sugarcane. PLA can degrade in months, under the right conditions, meaning there are no long-term environmental concerns of residue. PLA does not leach into foods while in use, so the material can be trustfully used as packaging or disposable tableware. PLA is safe and has no concern for environmental threat.

Polyurethane (PU)
PU is an ultra-durable form of plastic, used for items relating packaging and insulation products. PU has recyclability, but has some degree of degradability. The degradation of PU is relatively slow.
Additionally, PU has leachables during production, meaning caution while using and processing should be practice.

Consumers now have experience of consuming materials with an option or more environmentally friendly design or experience. These alternative materials can be some means to decrease health risks, and contribuited reduction of environmental pollution.
Is Polystyrene Recyclable?
In the plastic recycling symbol, the number “6” represents polystyrene (PS), however, it is not an easy material to recycle.

Polystyrene is bulky, particularly expanded polystyrene (EPS), but light. This makes transporting and storing it very expensive. Many polystyrene products, especially in food packaging, have food residues, grease, and other contaminants that make cleaning and disposal very expensive.
Not every recycling facility manages polystyrene, restricting recycling options in many areas.
There are a few ways to recycle polystyrene:
- Mechanical recycling: PS is collected, cleaned, crushed, and melted to be formed into new products.
- Chemical recycling: Polystyrene is decomposed into styrene monomers which are repolymerized into new polystyrene. This can also work with contaminated polystyrene, however, the process is very complicated and expensive.
- Compression recycling: Expanded polystyrene is compressed to minimize its size to decrease the cost of disposal and transportation.
Recycled polystyrene can be constructed into insulation, molds made from plastics, and garden furniture, and building materials can also be made.
Recycling polystyrene is possible, the recycling rate is slowed down by high price and processing complications, in order to keep the recycling rate higher, we need to develop more recycling places and better recycling efficiency.

Conclusion
Polystyrene is considered relatively safe under normal conditions. However, when it is exposed to high temperatures or in contact with acidic or fatty foods, it may release some chemicals that are harmful to health.
To reduce these potential health risks, we can take some simple measures, such as avoiding using polystyrene products in high temperature environments, not heating them in microwaves, and minimizing contact with acidic or alkaline foods.
In this way, we can better protect the health of ourselves and our families while reducing the negative impact on the environment.

